
High Dose Steroids in Children with Stroke and Unilateral Focal Arteriopathy – PASTA (Paediatric Arteriopathy Steroid Aspirin) trial:
A prospective multicenter, parallel group, two-arm, randomized controlled, open-label clinical trial with blinded outcome assessment
Please find more information here.
Study Overview
Aim of the study
The aim of this study is to investigate whether high dose corticosteroids result in greater and faster improvement of focal stenosis in children with stroke and FCA and whether this intervention influences neurological and neuropsychological outcomes as well as quality of life.
Background
Arterial ischemic stroke (AIS) is a rare but devastating condition affecting 2-5/100,000 children/year. Children do not recover better than adults with 2/3 suffering long term neurological, cognitive and behavioral problems. The economic cost of stroke is substantial. Arteriopathy is identified as AIS etiology in 60-80% of previously healthy children and is the strongest predictor of recurrent events. 30-40% of these children will have a focal cerebral arteriopathy (FCA) of the inflammatory type. FCA-i in childhood is shown to be an inflammatory vessel wall pathology provoked by infections. This encourages treatment with steroids, despite lack of evidence. Retrospective analyses suggest improved outcome and less recurrence with steroid treatment.
Outcome
Primary outcome is change in FCA severity score (FCASS) from baseline to 1 month
Secondary outcome are clinical outcome at 1-3-6-12 month by PSOM (local investigation) and RRQ, modified RS and VABS by telephone interview (blinded outcome). At 12 month a neuropsychological examination as well as assessment of quality of life is performed.
Secondary outcome by neuroimaging will be the course or FCASS up to 6 month, volume of stroke by mod ASPECT, residual arteriopathy at 6 month and recurrence of stroke (clmmmically and imaging).
Safety outcomes will include adverse events and serious adverse events related to steroid treatment
Trial design
The proposed study is a prospective multicenter, parallel group, two-arm, randomized controlled, open-label clinical trial with blinded outcome assessment, comparing a high dose course of methylprednisolone/prednisolone plus standard of care with standard of care alone in children with unilateral arteriopathy and acute ischemic stroke.
Please check the key inclusion and exlcuion criteria below:

- Age > 6 months & < 18 years at time of stroke
- Randomisation possible within 48 hours of diagnosis
and maximum 96 hours after stroke onset - Unilateral arteriopathy with:
- Newly acquired neurologic deficits
- Specific neuroimaging (MRA) features of
– unilateral stenosis, or
– unilateral vessel irregularities within the CNS
- Females age ≥ 13: Negative pregnancy test
- Informed consent

- Previous stroke
- Known syndromal disorders, as e.g. Trisomy 21, Neurofibromatosis type 1
- Known genetic vasculopathies
- Moyamoya or sickle cell disease
- Small vessel cerebral vasculitis
- Bilateral arteriopathy
- Arterial dissection(s)
- Underlying systemic disorders, as lupus
- CNS angiitis due to infections
- Progressive large to medium childhood primary angiitis of the CNS (cPACNS )
- On steroid ttm at disease onset
- Contraindication to steroid treatment as immunodeficiency
- Inability to follow study procedures
- Participation in another interventional study within the 30 days preceding the indication stroke and during the present study
Treatment / Control group
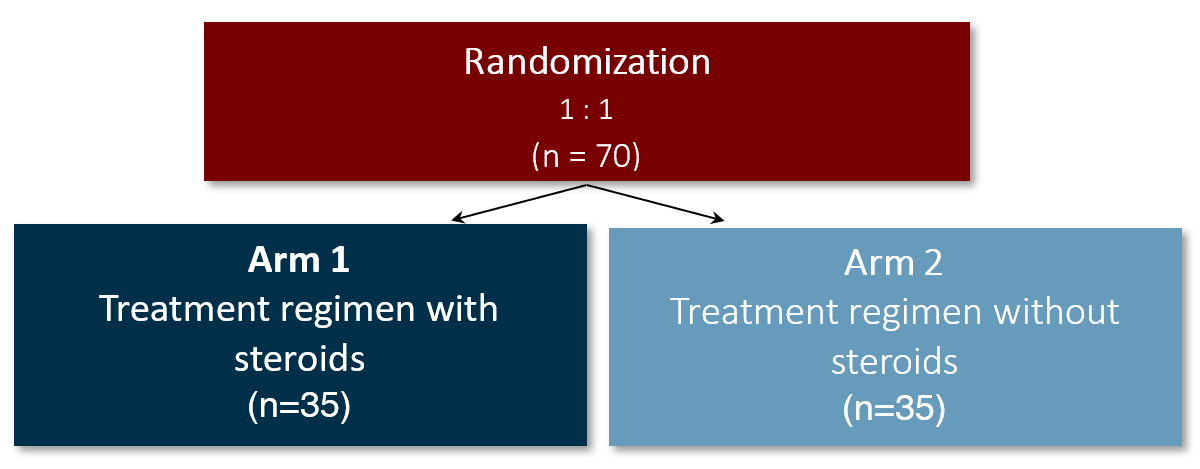
The experimental intervention consists of 3 days intravenous methylprednisolone at a dose of 30mg/kg BW (max. of 1000mg/dose) followed by a 4 week oral course of tapering oral prednisolone: week 1 and 2, oral prednisolone 1mg/kg/day (max 40mg/day); week 3 and 4, oral prednisolone 0.5mg/kg/day (max 20mg/day).
Both arms will receive standard of care for children with FCA-I, including Aspirin treatment
Follow up
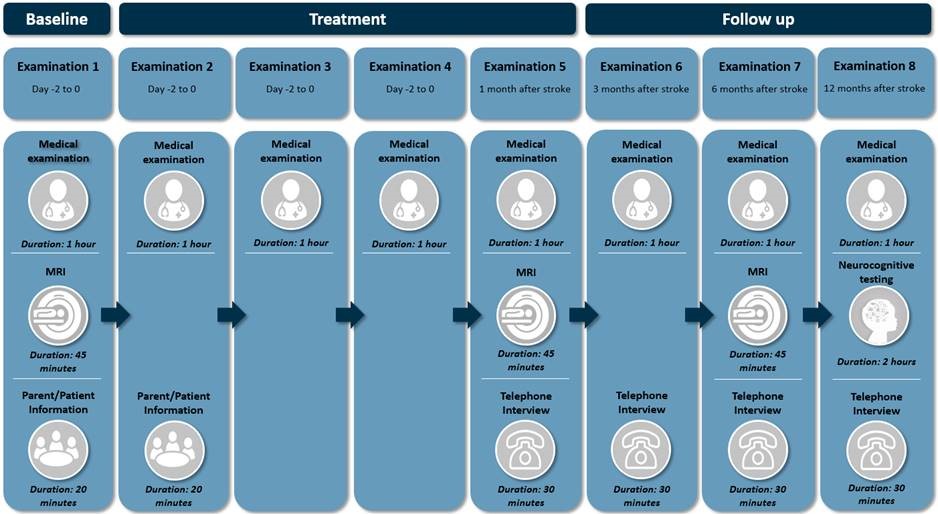
FCASS classification
The focal cerebral arteriopathy scaling system (=FCASS) has been developed to improve diagnostic criteria and to better document the typical course of initial worsening followed by improvement in FCA-i. This scaling system has been validated Prof. H. Fullerton and her team and was also evaluated in the Swiss cohort of children with FCA-i. Both analyses show the typical course of the arteriopathy, but have also shown, that the degree of stenosis as expressed by FCASS is correlated with stroke volume and neurological outcomes of these children.
ped-NIHSS
The ped-NIHSS is a tool to asses severity of stroke in childhood. The video was created specifically to assist the PASTA study investigators in performing the ped-NIHSS in as standardized a manner as possible.
PSOM
PSOM is an instrument to assess outcome after stroke in childhood. The video was created specifically to assist the PASTA study investigators in performing the PSOM in as standardized a manner as possible.
Funding
The PASTA trial is funded by the Swiss National Foundation of Research
Impact
This investigator-initiated international trial will give evidence to the treatment with high dose steroids in children with acute stroke due to a focal arteriopathy of the inflammatory type. Besides treatment trials in treatment of sickle cell disease this is the first randomized treatment trial worldwide in childhood stroke